CIBOR advances aerospace materials in medical applications
CFRP in surgical instruments and orthopedic implants gets assistance from Wichita R&D center.
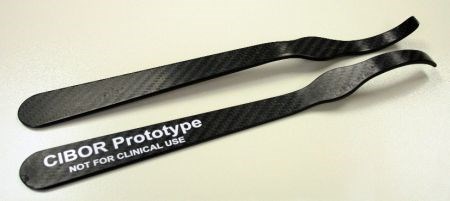
CIBOR performs R&D on a variety of bioscience and medical applications, including composite surgical instruments, joint implants and orthopedic implants which help stimulate bone growth.
SOURCE: CIBOR.
The Center of Innovation for Biomaterials in Orthopedic Research (CIBOR) at the National Institute for Aviation Research (NIAR, Wichita, KS, US) seeks to bridge the gap between the aviation and medtech sectors, applying aerospace materials & process knowledge to the design and development of medical devices, specifically in orthopedics. CIBOR performs R&D on composite materials for a wide variety of bioscience and medical applications, including orthopedic implants and surgical instruments. Composites have benefits in medical applications which could enable significant improvements in battlefield and sports medicine, reduced cost, decreased time and invasiveness of orthopedic surgical procedures, and improved rehabilitation and long-term outcomes for orthopedic patients.
CIBOR draws upon the composites expertise of Wichita’s well-established aviation manufacturing community and NIAR to deliver global leadership in the design and fabrication of composites-based medical technology. It also works with local manufacturers seeking to diversify their composites manufacturing outside of aerospace. “CIBOR is very unique,” explains senior research engineer Kim Reuter, “we have engineers that are excellent at composite laminate design, but also have an outstanding biology team that can perform biocompatibility studies and bone growth analyses.”

CFRP's advantages in orthopedic surgical instruments include radiolucency and surfaces that do not scratch implants as readily as stainless steel. They must also be very strong to resist the forces applied during orthopedic surgeries. SOURCE: CIBOR.
Composites in sterilized surgical instruments
“The advantage of composites over stainless steel in orthopedic surgical instruments is radiolucency,” explains Kim Reuter, senior research engineer at CIBOR. “In other words, except for locating markers, they are not visible on x-ray or fluoroscopy equipment.” She notes that surgeons actually asked instrument manufacturers for this. “It is needed, for example, when an orthopedic surgeon is trying to line up a screw with a fixation device implanted within a long bone.” Stainless steel shows up as bright white on a fluoroscope (real-time x-ray), obscuring the surgeon's view. In that case, the medical team must take all of the instruments out, observe the site of interest, and then put them all back. “This is time-consuming and increases risk to the patient,” Reuter adds. Carbon fiber reinforced plastic (CFRP) instruments, however, do not show on the fluoroscope and allow for a clear view of the anatomy without removal of the instruments.
Another important driver for CFRP is for instruments that do not scratch implants. “Knee implants are designed for fatigue, and thus are highly polished,” says Reuter. “The bearing surfaces must be smooth, as they form the articulating surface of the joint. If you scratch these, you create a crack initiator.” Reuter notes that scratching these surfaces is easy to do with stainless steel instruments. And yet, these surgical tools must be very strong. “Surgeons need durable, strong instruments that can withstand the forces applied during surgery as well as the harsh sterilization environment before and after surgery.” For this reason, CIBOR looked at continuous reinforcements, mostly woven and unidirectional tape. “The composite instruments are a little thicker but almost the same dimensions as the stainless steel. The surgeons said they felt just as stiff but lighter weight.”
One further requirement, however, is that instruments must maintain their mechanical performance properties despite repeated sterilization cycles between surgical procedures. According to Andi Meyer, also a senior research engineer at CIBOR, the most common process is steam sterilization: An autoclave is filled with instruments, sealed and then filled with forced steam under high pressure to destroy microorganisms and denature enzymes and proteins. Time and temperature vary, but a one-hour cycle is common, 15 minutes of which may be at the highest pressure and temperature.
Meyer has recently completed a series of steam sterilization tests, aimed at understanding how a variety of composite materials may perform as surgical instruments after hundreds of steam sterilization cycles. Although a number of studies have reported that carbon fiber (CF) reinforced polyetheretherketone (PEEK) performs well under repeated steam sterilizations, Meyer says these mostly involved neat PEEK or chopped CF reinforced PEEK. “My results were different,” explains Meyer, “because I used stronger and stiffer materials more standard to aerospace, such as continuous carbon fiber thermoset and thermoplastic laminates, including PEEK.”
Meyer tested their properties, pre-sterilization, and again, after 200, 400, 600, 800 and 1,000 steam sterilization cycles. Meyer explains, "the best material for an instrument depends on what the primary driver is — e.g., is the main goal a cheap instrument or a very long-lasting one? — and also on the manufacturer. For example, PEEK is expensive and many of our local manufacturers prefer not to work with it.” She says that CF/epoxy tested after 1,000 steam sterilization cycles showed 84% of original strength, while CF/PEEK showed only 54%. However, the appearance of the CF/epoxy was significantly more degraded than the CF/PEEK. "This study had surprising results that discourages generalization of material properties," says Meyer. "It showcases the need for experienced composite engineers to design medical products appropriately."
She notes that, "Not all CFRP can handle the hot-wet environment of steam sterilization. Designing for high strength applications and testing materials in harsh environments is second nature for composite experts in the aerospace industry, but unfortunately, some composite medical devices are being introduced into the market without evaluating all operational conditions." Meyers asserts that improperly designed composites are creating a negative impression, "which adds to the challenge of bringing composites into the medical industry."
|
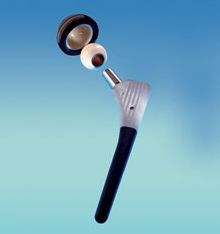
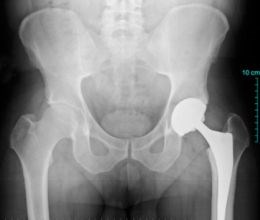
Carbon fiber/PEEK hip implants (left) are being developed with the goal to reduce the need for revision surgery (repeat hip joint replacement) in 15 years or less due to complications from bearing surface wear. SOURCE: (left) Composites in daily life, composites.ugent.be, (right) “A PEEK into the Future of Hip Implant Bearing Surfaces,” QMED.com.
Revision-free implants require new materials and test methods
This CIBOR research was sponsored by an orthopedic manufacturer who wanted to research CFRP in hip and knee implants. Though used in spinal implants for over two decades, and gaining acceptance in plates and nails for fracture fixation, this composite material is still in exploratory stages for total joint replacements like knees and hips.
Past CIBOR chief scientific officer, Dr. Paul Wooley, reported that although hip and knee implants are designed to have an estimated 15-year lifespan, they often fail quite short of that. He adds that now 30% of patients in their 50s and 55% of patients younger than 50 will require revision surgery — replacement of the implant with another implant — before the 15-year lifecycle expires. Revisions are also expensive, costing 41% more than the primary surgery, with a hospital stay that is typically 2 days longer, a slower recovery and a 32% higher rate of complications. Patients are also receiving implants at a younger age and demanding higher performance. Thus, a longer-lasting, durable hip or knee implant that does not require revision procedures is indeed a Holy Grail.
CIBOR senior research engineer Andi Meyer explains that there is significant mechanical stress during implant service life. Bearing surface wear and resulting debris are widely regarded as primary culprits for issues such as osteolysis (bone tissue destruction), loosening leading to implant failure.
CFRP reportedly offers potential for joint replacements that last longer than 15 years. “Strong and stiff implants made of traditional metals shield the bone from stress, whereas CFRP materials can be tailored to match stiffness of the bone" says Meyer, "this allows the bone to be subjected to stress so it does not become weak and start to atrophy.” The latter happens with metal implants, which means revision surgery. “Since you lose bone stock with each revision,” she says, “eventually, revision becomes impossible.” (This is why joint replacements are typically avoided for young people.)
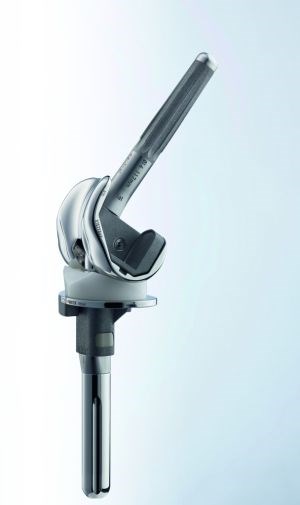
Aesculap, a division of healthcare supplier B. Braun Melsungen, is using CF/PEEK in its FDA-approved EnduRo knee implant. Aesculap worked with Invibio Biomaterial Solutions, which was formed in 2011 as a wholly owned subsidiary of Victrex plc to focus exclusively on the medical device market. SOURCE: Plastics Today and Aesculap.
According to Meyer, a composite implant can be designed to flex more and share the load with surrounding bone so there is less bone loss. “The problem,” says Meyer, “is the ASTM test methods for these implants were not designed for this different type of load-sharing performance offered by composite materials.” In fact, CFRP’s improved flexibility actually precluded it from passing the legacy performance tests, because the standards used to evaluate implants were written for metal.
“We have recommended that the industry consensus test standards be rewritten to define thresholds required for using CFRP or other materials not currently used in replacement devices,” says Meyer. “However, when we went to the orthopedics capital — Warsaw, Indiana — and talked to the major players, we discovered a lack of interest in collaboration on this topic. To our knowledge, no progress has been made on revising the test standards.”
Even so, carbon fiber composite implants are proceeding into the market. A 2012 report in Plastics Today touted CF/PEEK’s successful performance in a total knee arthroplasty (TKA) and a 2014 research article looked at 62 TKA patients receiving this device, showing successful results, though early still, and for a relatively small cohort. A 2015 literature review also showed CF/PEEK to have potential wide-scale success in joint and other implants, though it admitted its span of 24 articles was limited.
CIBOR has also made significant progress in testing carbon foam materials as aids in bone growth and regeneration. Be sure to read about it in our upcoming June issue’s feature article.
Related Content
Filament winding increases access to high-performance composite prostheses
Steptics industrializes production of CFRP prostheses, enabling hundreds of parts/day and 50% lower cost.
Read MoreComposites end markets: New space (2025)
Composite materials — with their unmatched strength-to-weight ratio, durability in extreme environments and design versatility — are at the heart of innovations in satellites, propulsion systems and lunar exploration vehicles, propelling the space economy toward a $1.8 trillion future.
Read MorePlant tour: Teijin Carbon America Inc., Greenwood, S.C., U.S.
In 2018, Teijin broke ground on a facility that is reportedly the largest capacity carbon fiber line currently in existence. The line has been fully functional for nearly two years and has plenty of room for expansion.
Read MoreCarbon fiber, bionic design achieve peak performance in race-ready production vehicle
Porsche worked with Action Composites to design and manufacture an innovative carbon fiber safety cage option to lightweight one of its series race vehicles, built in a one-shot compression molding process.
Read MoreRead Next
Post Cure: CFRP mainframe, modern manufacturing techniques pioneer next-generation rigid airships
Advanced composites enable the revival of rigid airships in LTA Research's 400-foot-long Pathfinder 1.
Read MoreBonded fastening meets the digital factory
Automation and XR tools aim to scale adhesive fastening for composites at next-gen aerospace production rates.
Read MoreKaneka BMI material attains >25% lead time, >20% tool cost reductions in Janicki evaluations
Process evaluations find that novel BMI polymer chemistry addresses longstanding manufacturing challenges of BMI tooling prepregs while maintaining high temperature performance and durability.
Read More






















