Polyester resins: The basics
Polyester resins are widely used resin systems. Learn more about their chemical makeup and how they are used in the composites industry.
Editor’s note: This content was originally published on NetComposites.com. NetComposites was acquired by CompositesWorld’s parent company, Gardner Business Media, in February 2020.
Polyester resins are the most widely used resin systems, particularly in the marine industry. By far the majority of dinghies, yachts and workboats built in composites make use of this resin system.
Polyester resins such as these are of the “unsaturated” type. Unsaturated polyester resin is a thermoset, capable of being cured from a liquid or solid state when subject to the right conditions. It is usual to refer to unsaturated polyester resins as “polyester resins,” or simply as “polyesters.” There is a whole range of polyesters made from different acids, glycols and monomers, all having varying properties.
There are two principle types of polyester resin used as standard laminating systems in the composites industry. Orthophthalic polyester resin is the standard economic resin used by many people. Isophthalic polyester resin is now becoming the preferred material in industries such as marine, where its superior water resistance is desirable.
The figure below shows the idealized chemical structure of a typical polyester. Note the positions of the ester groups (CO – O – C) and the reactive sites (C* = C*) within the molecular chain:
Most polyester resins are viscous, pale-colored liquids consisting of a solution of a polyester in a monomer which is usually styrene. The addition of styrene in amounts of up to 50% helps to make the resin easier to handle by reducing its viscosity. The styrene also performs the vital function of enabling the resin to cure from a liquid to a solid by “cross-linking” the molecular chains of the polyester, without the evolution of any by-products. These resins can therefore be molded without the use of pressure and are called “contact” or “low-pressure” resins. Polyester resins have a limited storage life as they will set or “gel” on their own over a long period of time. Often small quantities of inhibitor are added during the resin manufacture to slow this gelling action.
For use in molding, a polyester resin requires the addition of several ancillary products. These products are generally:
- a catalyst,
- an accelerator, or
- additives for thixotropic properties, pigment, filler or chemical/fire resistance.
A manufacturer may supply the resin in its basic form or with any of the above additives already included. Resins can be formulated to the molder’s requirements ready simply for the addition of the catalyst prior to molding. As has been mentioned, given enough time an unsaturated polyester resin will set by itself. This rate of polymerization is too slow for practical purposes and therefore catalysts and accelerators are used to achieve the polymerization of the resin within a practical time period. Catalysts are added to the resin system shortly before use to initiate the polymerization reaction. The catalyst does not take part in the chemical reaction but simply activates the process. An accelerator is added to the catalyzed resin to enable the reaction to proceed at workshop temperature and/or at a greater rate. Since accelerators have little influence on the resin in the absence of a catalyst they are sometimes added to the resin by the polyester manufacturer to create a “pre-accelerated” resin.
The molecular chains of the polyester can be represented as follows, where “B” indicates the reactive sites in the molecule:

With the addition of styrene “S,” and in the presence of a catalyst, the styrene cross-links the polymer chains at each of the reactive sites to form a highly complex three-dimensional network as follows:
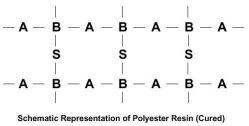
The polyester resin is then said to be “cured.” It is now a chemically resistant (and usually) hard solid. The cross-linking or curing process is called “polymerization.” It is a non-reversible chemical reaction. The “side-by-side” nature of this cross-linking of the molecular chains tends to means that polyester laminates suffer from brittleness when shock loadings are applied.
Great care is needed in the preparation of the resin mix prior to molding. The resin and any additives must be carefully stirred to disperse all the components evenly before the catalyst is added. This stirring must be thorough and careful as any air introduced into the resin mix affects the quality of the final molding. This is especially so when laminating with layers of reinforcing materials as air bubbles can be formed within the resultant laminate which can weaken the structure. It is also important to add the accelerator and catalyst in carefully measured amounts to control the polymerization reaction to give the best material properties. Too much catalyst will cause too rapid a gelation time, whereas too little catalyst will result in under-cure.
Coloring of the resin mix can be carried out with pigments. The choice of a suitable pigment material, even though only added at about 3% resin weight, must be carefully considered as it is easy to affect the curing reaction and degrade the final laminate by use of unsuitable pigments.
Filler materials are used extensively with polyester resins for a variety of reasons including:
- to reduce the cost of the molding,
- to facilitate the molding process, or
- to impart specific properties to the molding.
Fillers are often added in quantities up to 50% of the resin weight, although such addition levels will affect the flexural and tensile strength of the laminate. The use of fillers can be beneficial in the laminating or casting of thick components where otherwise considerable exothermic heating can occur. Addition of certain fillers can also contribute to increasing the fire-resistance of the laminate.
For the latest on poly/vinyl ester resins, visit compositesworld.com/zones/poly-vinyl-esters.
Related Content
Troubleshooting thermoplastic composite stamp forming
Understand the basic science of TPC stamp forming, a manufacturing process steadily gaining momentum in aerospace and mobility applications thanks to its rapid forming, short cycle times and automated methods.
Read MoreThermoplastic composite fabrication: Thermal processing
Establish a proper thermal cycle during TPC rapid forming and achieve reproducible, successful parts through key material selection and process method understanding.
Read MoreComposites end markets: Energy (2025)
Despite political and supply chain challenges, renewable and nuclear energy continue to grow in use. Composite materials enable current and future energy technologies across sectors.
Read MoreUnderstanding the difference between bonding and welding
Composites bonding and welding are two similar but distinct processes that overcome challenges related to fasteners or drilling. Here are some resources to get you started.
Read MoreRead Next
From PMCs to sandwich composites: Tracing the path of test method standardization
Over the decades, progression of PMC and sandwich composite test method development and standardization has been shaped by the requirements of the composites industry.
Read MoreThermoplastic composite materials and processing interactions
Selection of product material formats and their interactions with various process methods heavily influence a final TPC part’s properties and fabrication options.
Read MoreCutting engine weight via thermoplastic composite guide vanes
Greene Tweed replaces metal stator vanes with its DLF material co-molded with a metal leading edge that meets performance, cost and high-rate production targets while cutting 4 kg per engine.
Read More





















