Designing-in Corrosion Resistance
The most cost-effective way to prevent corrosion is to select corrosion-resistant materials.
There is general agreement that corrosion is one of the more serious problems plaguing military systems. A recent, federally funded study has estimated the cost to the U.S. Department of Defense (DoD) at over $20 billion (USD) per year (Corrosion Costs and Preventive Strategies in the United States, G Koch, M. Broyers, N. Thompson, Y.P. Virmani and J. Payer, co-authors). But the unanimity of opinion generally stops there. Within the DoD, there are tremendously disparate perspectives on what corrosion even represents. To some, it is strictly a maintenance problem -- an inevitable part of doing business. To others, corrosion is a chemical phenomenon that occurs in most metals, but no connection is made to real-world impacts. In most cases, these views are highly compartmentalized, lacking the "big picture" of how corrosion affects the entire life cycle of a structure, from design through retirement and disposal. Though the scope of this article is military systems, the problems and solutions outlined here apply equally well to the manufacture of components and structures in any potentially corrosive environment.
All too often, corrosion begins on the drawing board. Designers often overlook steps needed to ensure adequate corrosion resistance in their designs. Systems are designed to meet performance goals where strength, weight, thermal and electrical requirements are primary technical considerations. Other important attributes, such as corrosion resistance and environmental compatibility, typically receive far less attention. Without an upfront analysis to assess and address potential corrosion issues, many problems are discovered only after the system has been put into service. Correcting unanticipated corrosion problems during the operational phase of an asset's life cycle can be very costly. In some cases, it may be impossible to restore a system to its original state without replacing problematic components or structures.
Those responsible for design and acquisition of weapon systems (aircraft, land systems, ships, munitions, etc.), support systems (trucks, cargo planes, supply ships, etc.) and infrastructure (buildings, storage tanks, piping, water treatment plants, piers, etc.) must recognize that corrosion is an important risk factor among many requiring active management early in the design phase. A fielded system can be maintained in its original, corrosion-free state after entering service far more easily and at a greatly reduced cost if excessive corrosion is prevented from occurring in the first place. The best way to prevent or minimize corrosion throughout a system's lifecycle is to select corrosion-resistant materials, such as composites, during the design process. Since sound material selection decisions can greatly minimize the incidence and severity of corrosion, but usually cannot eliminate it entirely, designers should therefore be aware of and employ an array of innovative corrosion prevention and control (CPC) technologies to augment that protection. Components also must feature "corrosion-smart" designs developed with maintenance strategies in mind.
UNDESTANDING SYSTEM LIFE CYCLE
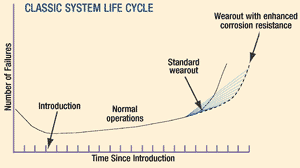
While this undertaking may seem challenging (and more costly up-front), the long-term benefits more than justify the investment. The "Classic System Life Cycle" graph on p. 31 illustrates the life cycle of a system as it relates to component failure. Known as the "bath-tub curve," the graph demonstrates the various rates at which components may fail during their service life. Generally, this addresses the sum total of all failures, including those induced by corrosion. However, it's reasonable to assume that a similar curve would result from failures due to corrosion alone.
As illustrated on the curve, the three phases of a system life cycle consist of 1) introduction of the system into service, 2) normal operational use, and 3) wear-out. During the introductory phase the manufacturing defects are identified and corrected, which results in a higher number of failures initially, followed by a steady decline. For systems designed with insufficient attention to corrosion, this initial phase corresponds to the identification and mitigation of unexpected corrosion. The bulk of the life cycle is spent as the system operates normally (the second phase) with only routine maintenance and repairs. It is very important to properly maintain the system during this phase by employing corrosion preventative measures. If steps are not taken, the system will corrode, thus accelerating the initiation of the third phase, where the number of failures and associated maintenance actions, including component replacement, begin to increase steadily as the system reaches its maximum operational life. Proper materials selection during the design phase, followed by the use of appropriate CPC practices during service, will delay the onset of wear-out and enable the affordable extension of a system's life, delaying replacement of systems.
There is a direct correlation between the number of failures shown in the life-cycle graph and total ownership cost. As the amount of failures (and consequent extraordinary corrosion preventative measures) rise, so does the total cost. In systems designed without inherent corrosion resistance, it can be expected that the wear-out phase will be reached in a shorter period of time. Consequently, an entirely new system will have to be purchased earlier than scheduled or, alternatively, extensive maintenance will be necessary to keep the existing system in operation; both of which would carry significant costs.
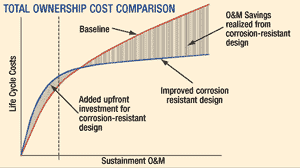
It's difficult to project a definitive return on investment resulting from increased attention to corrosion control during system design. Currently, there are no available management tools (software) that can quantify the total ownership cost savings and calculate the return on investment that would result from increased attention to CPC issues during the design phase. Nevertheless, it is quite easy to understand intuitively that a system designed with inherent corrosion resistance will last longer than one without. The life-cycle graph addresses both situations, with the dashed lines indicating a longer service life for the system designed with corrosion resistance in mind. Take, for example, a hypothetical weapon system that, due to its inherent corrosion resistance, has a life span that is two years longer than a similar (baseline) system. If the baseline life was ten years and the total acquisition cost was $1 billion (USD), the return on investment due to delayed acquisition of a follow-on system would be $200 million. In reality, the cost savings would be even higher than this projection, because this example totally ignores operations and maintenance (O&M) savings. In the "Total Ownership Cost Comparison" graph (below), we see two hypothetical systems, one designed with inherent corrosion resistance and the other without. Acquisition costs for the former are shown to be higher, due to increased engineering time and potentially more expensive materials. However, over the system's life, these costs are more than recovered in O&M cost savings, such that the total ownership costs of the corrosion resistant system will be lower than the baseline system.
PROPER MATERIALS SELECTION ENSURES CORROSION RESISTANCE
A thorough and realistic consideration of corrosion prevention and control during the materials selection phase of the design process is key to developing systems that will age in a predictable and affordable fashion. This requires a firm understanding of the system's operating environment, that is, the conditions it may be exposed to while in service. This step isn't as simple as it appears. A ship floating in the ocean obviously will be exposed to the corrosive effects of seawater. A jet engine turbine blade, of course, will be subject to extreme temperatures. In reality, however, these and other systems experience a variety of simultaneous environmental conditions, many of them not so obvious. For instance, systems often contain fluids and chemicals that, while necessary for its components to operate, are nevertheless very corrosive. Ordinary cleaning chemicals and hydraulic fluids are potential sources of contamination that can contribute to corrosion. An operating environment isn't a single condition, therefore, but rather a combination of factors that work in concert -- operating temperature and humidity, salinity, mechanical loading as well as exposure to chemicals, fuel, pollutants and biological organisms. Designers must take a step back and gain a firm understanding of all environmental factors that can influence corrosion before selecting the construction materials.
In addition to the operating environment, designers must also consider environmental conditions that occur during storage or transportation. Systems can experience corrosive environments during transportation that are far more severe than their operational conditions, yet designers can easily overlook the threat of transportation-induced corrosion, since systems experience only brief periods being transported from one part of the globe to another. While some weapons, like air-launched missiles, are stored in controlled-humidity containers, which help keep these systems in their pristine, uncorroded state, other weapons, such as gravity bombs, may be exposed to extremely high humidity levels while in their protective shelters, corroding so severely that they may become useless and need to be replaced.
TESTING AND RESOURCES
One of the reasons why selecting corrosion-resistant materials is a challenging process is that corrosion data aren't usually available in forms that are immediately and directly related to the respective environment of the system or structure. Because of the extremely large number of available materials, (including variants subjected to different fabrication processes, and environmental conditions), it is too costly and nearly impossible to test all the combinations of materials and environments. The result is that it can be very difficult to find completely relevant data to substantiate decisions. Materials scientists over the years have devoted significant resources to corrosion testing and analysis. Accelerated testing using salt spray or controlled humidity and temperature chambers are often used to investigate a material's potential to corrode or how well a CPC technology will function. Unfortunately, these tests don't replicate true operational conditions, nor do they account for the synergistic effects of other contributing factors such as atmospheric pollutants or chemical exposure.
Perhaps the best source of information to address both the expected operational environment and the potential for corrosion problems is to consult the existing literature. Such a review can determine whether there is documented field service experience on a legacy system similar to the one being designed. Natural aging information for a system or structure operated within the same (or similar) environment to one being designed can provide excellent insight as to what to expect. In addition, if materials used on the older system have shown the potential to corrode, then there are lessons learned that can be used to preclude incorporation of problematic materials in new systems.
It can be tempting to minimize predicted corrosion problems by relying solely upon legacy technology. It is common practice in industry to build new systems from the same materials as their predecessors. And, in some situations, this may be an entirely acceptable approach, but it is fraught with the risk of overlooking new materials and technologies, thus representing a lost opportunity. New materials are often innovative in nature, allowing a designer to exploit their improved properties to provide performance advantages over a legacy or competing system. Therefore, in situations where no laboratory or in-service data are available, designers shouldn't be deterred from the use of new materials. The known environmental conditions within an existing system of similar nature can be used as a guide to project whether future corrosion problems can be expected for new materials. Such an assessment can lead engineers to develop test protocols that will quantify the degree of corrosion resistance inherent in the new material. It also may be possible to employ effective CPC strategies with a new material to provide the necessary corrosion protection. The use of CPC methods, such as chemical treatments, paints, platings and cathodic protection, must be planned and not left as an afterthought, or else it is highly likely that maintenance problems will plague the system throughout its service life.
DESIGN APPROACHES
Even with proper materials selection, designers can unintentionally exacerbate the likelihood of corrosion by creating conditions that favor its occurrence. For example, if drainage holes are not included in a structure subjected to rain or wash water, liquid can become trapped and accelerate the corrosion process in an entirely unanticipated location and fashion. Other considerations include using materials that won't wick moisture. It's important to avoid, if at all possible, the use of wood, paper, cardboard, open cell foams, and sponge rubbers in systems that operate in wet or humid environments. These materials tend to retain water and, consequently, function as reservoirs for adjacent materials that may be susceptible to corrosion.
Another key design consideration involves junctions between adjacent components. Known as faying surfaces, these interfaces can see relative movement between parts sufficinet to wear away protective surface layers at the joint, exposing the underlying material directly to a corrosive environment. To protect faying surfaces, proper sealing materials (tapes, films, sealing compounds) and primers must be applied.
The intimate contact of two adjacent materials can be the causative factor for another corrosion mechanism, galvanic corrosion. One of the eight main forms of corrosion (see "Corrosion Mechanisms" p. 32), it results when dissimilar metals come in contact with each other and are exposed to corrosive conditions. One of the best ways to prevent these materials from corroding further is to electrically insulate them through the use of coatings at the interface between them. A nonconductive coating will prohibit electrons from moving between the two materials, thus stopping the oxidation process.
Another important aspect of design is maintenance access. It is vitally important that inaccessible areas be minimized so that maintenance personnel can both inspect the areas for corrosion and reapply CPC compounds or replace com- ponents if necessary. For those situations where inaccessible areas are unavoidable, then it is even more important that a proper upfront analysis be conducted to ensure that corrosion resistance can be sustained to preclude unanticipated and costly damage after the system is fielded.
When analyzing maintenance access requirements, designers should consider that nondestructive evaluation techniques will be needed to detect hidden corrosion at some point during the operational use of the system. The structure must be designed to accommodate the necessary testing apparatus to preclude hidden corrosion from creating an unsafe or unreliable system.
DESIGN PITFALLS
Unfortunately, designers seldom have a strong background in or understanding of corrosion or its prevention and control. Most view corrosion as a single process and thus are unaware that highly accelerated, localized corrosion mechanisms even exist. It also is a mistake to assume that two seemingly identical materials that may have the same composition (relative amounts of elemental constituents) but have been processed differently (e.g, different heat treatments), would exhibit the same corrosion rates. The potential for a part to corrode is often strongly influenced by the processes used to create it.
Another pitfall is that characteristic relationships used to predict corrosion rates are inappropriately applied. A designer who lacks the necessary understanding of corrosion may consider all corrosion phenomena a single process, and thus may be prone to misapplying an equation developed for a different corrosion form. For example, a well-understood and predictable process such as uniform corrosion has been characterized by several constitutive equations which predict degradation rates. Unsuspecting engineers could mistakenly apply such equations to a highly accelerated, localized form of corrosion such as pitting or crevice corrosion, characterized by extremely high corrosion rates in very small and often hidden locations. The rates of corrosion for these mechanisms vary widely from one case to another; hence they don't lend themselves at all to predictive methodologies. Engineers who do not understand these differences can, and often do, misapply equations which yields a strong potential for unexpected failures.
Although experimental data concerning corrosion abounds, it is often in inconsistent formats. Combined with the fact that corrosion rates are highly dependent upon usage environments, material composition, and processing history, a common mistake is to use data inappropriately or incompletely. A designer with the right intentions can easily misuse data to substantiate a design decision that may lead to entirely wrong conclusions. Another contributing factor is that corrosion data, especially that relating to natural aging, is scattered and available from many different sources. This type of data is seldom consulted and, consequently, design decisions don't fully benefit from the lessons learned. The net result in both cases is that a system, structure or component will possess far worse corrosion characteristics than initially believed or desired.
FINDING THE RIGHT BALANCE
For the uninitiated, this discussion might give the impression that corrosion cannot be prevented without conducting painstaking and extraordinary measures. It is important to point out, however, that the goal of materials selection isn't to eliminate corrosion in all circumstances, but to manage and minimize it. The challenge to effective corrosion prevention and control is to strike a balance to ensure adequate inherent corrosion resistance and ease of maintenance while at the same time balancing cost. Cost includes not only the design time required to analyze and select the most appropriate material, but also the material cost itself. In general, materials that are inherently corrosion-resistant are more expensive than those that are not. During the design process, engineers must choose materials that provide the best combination of performance, including corrosion resistance, that will ensure that systems will adequately perform their function over the intended lifespan within fiscal constraints.
Reducing risk on new systems has to be an important concern for all stakeholders in the acquisition process. Correcting corrosion problems before they occur, by way of the materials selection process, is the best strategy for the long run.
Editor's note: AMPTIAC is the Advanced Materials and Processes Technology Information Analysis Center, a DoD-sponsored group administrated by the Defense Technical Information Center (DTIC). AMPTIAC is preparing to publish a major, first-of-its-kind corrosion resource: A Program Management Guide for Selecting Materials. It will provide designers with guidance on how actually to select materials that will enhance corrosion prevention and control. For more information about the forthcoming publication, contact David H. Rose, AMPTIAC director, E-mail: amptiac@alionscience.com.
CORR0SION MECHANISMS
There are eight ways corrosion can attack a metallic surface -- eight good reasons why designers, military and nonmilitary, should consider specifying composites.
Uniform attack. This most common form attacks an exposed surface uniformly, and its rate can be predicted via tests.
Galvanic corrosion occurs when two different metals or alloys (or certain other materials, such as carbon fiber and a metal) come in contact with each other when a corrosive substance is present. An electrochemical process takes place in the contact region, with one material acting as a cathode and therefore protected against oxidation at the expense of the other material, which becomes the anode and is consumed.
Crevice corrosion becomes active when a corrosive substance is trapped in a narrow gap between two components. As the reaction proceeds, the concentration of the corrosive agent increases, so the reaction proceeds at an ever-increasing rate.
Selective leaching results when one element from a solid alloy is preferentially removed through a corrosion process, typically through exposure to aqueous acids. The most common example is when zinc is removed from brass alloys, but aluminum, iron, cobalt and chromium are susceptible as well.
Intergranular corrosion occurs when the grain boundaries in a polycrystalline metal are preferentially attacked. A number of factors can make an alloy (e.g., austenitic stainless steel) susceptible to this type, including the presence of impurities and enrichment or depletion of one of the alloying elements (e.g., chromium) in the grain boundary area. One form of intergranular corrosion is weld decay.
Pitting corrosion. Almost always caused by chloride and chlorine containing ions, this form is very destructive (especially to stainless steel), because failure can result from a small percent weight loss of the actual structure. The pits have diameters less than or equal to their depths and, as they grow, can perforate the material's thickness.
Erosion corrosion results when one medium moves relative to another fixed medium (e.g., a liquid or slurry flowing through a pipe). A related form, fretting, occurs when two materials are in contact and a great degree of relative motion, such as vibration, exists between the two. This can erode anti-corrosion coatings, thus initiating corrosion.
Stress corrosion results when a material under tensile stress is exposed to a corrosive environment. The combination of these factors initiates cracks in the stressed component.
Related Content
Plant tour: Middle River Aerostructure Systems, Baltimore, Md., U.S.
The historic Martin Aircraft factory is advancing digitized automation for more sustainable production of composite aerostructures.
Read MoreMaterials & Processes: Composites fibers and resins
Compared to legacy materials like steel, aluminum, iron and titanium, composites are still coming of age, and only just now are being better understood by design and manufacturing engineers. However, composites’ physical properties — combined with unbeatable light weight — make them undeniably attractive.
Read MoreNatural fiber composites: Growing to fit sustainability needs
Led by global and industry-wide sustainability goals, commercial interest in flax and hemp fiber-reinforced composites grows into higher-performance, higher-volume applications.
Read MoreMaterials & Processes: Resin matrices for composites
The matrix binds the fiber reinforcement, gives the composite component its shape and determines its surface quality. A composite matrix may be a polymer, ceramic, metal or carbon. Here’s a guide to selection.
Read MoreRead Next
CW’s 2024 Top Shops survey offers new approach to benchmarking
Respondents that complete the survey by April 30, 2024, have the chance to be recognized as an honoree.
Read MoreFrom the CW Archives: The tale of the thermoplastic cryotank
In 2006, guest columnist Bob Hartunian related the story of his efforts two decades prior, while at McDonnell Douglas, to develop a thermoplastic composite crytank for hydrogen storage. He learned a lot of lessons.
Read MoreComposites end markets: Energy (2024)
Composites are used widely in oil/gas, wind and other renewable energy applications. Despite market challenges, growth potential and innovation for composites continue.
Read More















.jpg;maxWidth=300;quality=90)